Dilip Ghosh, PhD, FACN, nutriConnect07.01.15
Because of the unique health benefits and relatively low side effects, natural products such as food/dietary supplements, nutraceuticals and herbal medicines have been gaining popularity all over the world.
The gap between the popularity of these remedies and the frequently weak scientific basis of their use is striking. In reality, the efficacy and true frequency of side effects for most herbal medicine products is not known because the majority have not yet been tested in large clinical trials and because pharmacovigilance systems are much less extensive than those in place for pharmaceutical products. In contrast to the popularity of herbal medicinal products, physicians and consumers often have a very critical view of robust efficacy and safety profiles.
Genesis
“The use of traditional medicines, phytotherapeuticals, and dietary supplements should be based on quality, safety, efficacy, and consistency (QSEC)” (Cordell GA, Phytochemistry Letters, 2014). Traditional medicine was the only form of drug-based healing system in many parts of the world prior to introduction of aspirin in 1899. Over time, more than 50,000 biologically active plants have been described around the world (IUCN, 2007). The World Health Organization has recognized this need for an evidence base for all aspects of traditional medicine, making it clear in documents for many years (2002-2005, 2012) that the introduction of traditional medicine (TM) into healthcare systems must be based on evidence of QSEC.
The food/dietary supplements and herbal medicine products that are ingested are becoming a subject of increasing scrutiny and surveillance. Nowadays, the official regulatory agencies such as Food and Drug Administration (FDA), European Medicines Agency (EMA), European Food Safety Authority (EFSA), Therapeutic Goods Administration (TGA), Health Canada and so on ask industry for development of the food quality assurance program. In the past, there was not a widespread focus on the quality and safety of ingested health supplements. However, that does not mean that adulteration did not exist.
Seed-to-Patient Control
Due to the inherent variability of the constituents of herbal products, it is difficult to establish quality control parameters, and batch-to-batch variation. In the absence of reference standards for identification, variability can start from the collection of raw material, and increase during storage and further processing.
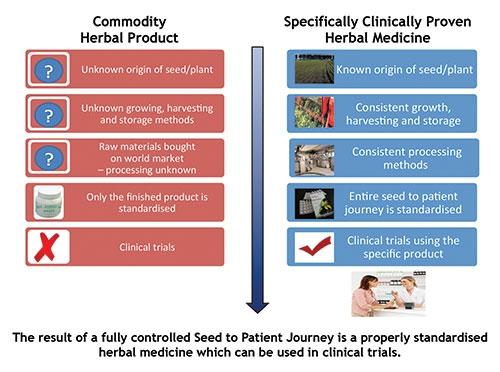
Generally, it is believed that the risk associated with herbal drugs is very low, but several reports on serious reactions are indicating the need for the development of safety profiles and stringent quality control systems for authentication, isolation, and standardization of herbal medicine (see Figure 1). Reliable and consistent quality is the basis for efficacy and safety of herbal medicinal products. Proper validation of herbs used in TM needs to be done for their promotion and development.
In conducting a clinical trial or during the preparation of food supplements, it is essential to clearly identify the plant component. The botanical identification is the first step of correct work. As a guidance tool, some experienced and tested approaches have been proposed to assist researchers in determining the quality and the safety of plant material (Zangara and Ghosh, 2014, “Clinical aspects of functional foods and nutraceuticals,” CRC Press).
Quality Issues
Excipients used in both pharmaceutical and nutraceuticals are no longer inert materials but they are effective and able to improve the characteristics of the products’ quality, stability, functionality, safety, solubility and acceptance among patients. They can change the medicament characteristics when they interact with the active ingredients (Abdellah A et al., 2015, Saudi Pharmaceutical Journal).
Since the introduction of the good manufacturing practice (GMP) regulations in the U.S. in 2007, the dietary supplements industry has continued to experience solid growth and now it’s part of the product approval system in many countries. Now the onus is on dietary supplement manufacturers/marketers to ensure that dietary supplements are safe and meet the quality standards that consumers and regulators are expecting. This can only be achieved through the rigorous application of in-house hazard analysis and critical control points (HACCP) and GMP systems from incoming raw material qualification and compliance to finished products (LeDoux et al., European Journal of Nutrition, 2015: 54).
The Way Forward
Several recent cases of adulteration and contamination of herbal medicinal products, which may have led to severe illness and possibly death in some cases, have revealed the necessity and urgency of identifying the plant material in botanicals and phytomedicines.
The older system of organoleptic (or identification through microscopic observation of plant parts) is not convincing, and plants are often misidentified. Recently, DNA-based methods have been applied to these products because “DNA is not changed by growth conditions unlike the chemical constituents of many active pharmaceutical agents,” (Moraes et al., 2015, Planta Medica).
The New York State attorney general recently called for the removal of certain herbal supplements from retail stores because DNA barcoding allegedly showed that most of the tested products did not contain the herbs listed on their labels. Although there is a strong counter-argument, DNA-based authentication of the ingredients incorporated into natural products is “perhaps the most efficient and economical method by which adverse effects of adulterants and plant or microbial contaminants may be minimized,” (Moraes et al 2015).
Dilip Ghosh, PhD, FACN
nutriConnect
Dilip Ghosh, PhD, FACN, is director of nutriConnect, based in Sydney, Australia. He is also professionally involved with Soho Flordis International, the University of Western Sydney, Australia, and is an Honorary Ambassador with the Global Harmonization Initiative (GHI). Dr. Ghosh received his PhD in biomedical science from University of Calcutta, India. He has been involved in drug-development (both synthetic and natural) and functional food research and development both in academic and industry domains. Dr. Ghosh has published more than 60 papers in peer-reviewed journals, and he has authored two recent books, “Biotechnology in Functional Foods and Nutraceuticals,” and “Innovation in Healthy and Functional Foods,” under CRC Press. His next book, “Clinical Perspective of Functional Foods and Nutraceuticals” is in press. He can be reached at dilipghosh@nutriconnect.com.au; www.nutriconnect.com.au
The gap between the popularity of these remedies and the frequently weak scientific basis of their use is striking. In reality, the efficacy and true frequency of side effects for most herbal medicine products is not known because the majority have not yet been tested in large clinical trials and because pharmacovigilance systems are much less extensive than those in place for pharmaceutical products. In contrast to the popularity of herbal medicinal products, physicians and consumers often have a very critical view of robust efficacy and safety profiles.
Genesis
“The use of traditional medicines, phytotherapeuticals, and dietary supplements should be based on quality, safety, efficacy, and consistency (QSEC)” (Cordell GA, Phytochemistry Letters, 2014). Traditional medicine was the only form of drug-based healing system in many parts of the world prior to introduction of aspirin in 1899. Over time, more than 50,000 biologically active plants have been described around the world (IUCN, 2007). The World Health Organization has recognized this need for an evidence base for all aspects of traditional medicine, making it clear in documents for many years (2002-2005, 2012) that the introduction of traditional medicine (TM) into healthcare systems must be based on evidence of QSEC.
The food/dietary supplements and herbal medicine products that are ingested are becoming a subject of increasing scrutiny and surveillance. Nowadays, the official regulatory agencies such as Food and Drug Administration (FDA), European Medicines Agency (EMA), European Food Safety Authority (EFSA), Therapeutic Goods Administration (TGA), Health Canada and so on ask industry for development of the food quality assurance program. In the past, there was not a widespread focus on the quality and safety of ingested health supplements. However, that does not mean that adulteration did not exist.
Seed-to-Patient Control
Due to the inherent variability of the constituents of herbal products, it is difficult to establish quality control parameters, and batch-to-batch variation. In the absence of reference standards for identification, variability can start from the collection of raw material, and increase during storage and further processing.
Generally, it is believed that the risk associated with herbal drugs is very low, but several reports on serious reactions are indicating the need for the development of safety profiles and stringent quality control systems for authentication, isolation, and standardization of herbal medicine (see Figure 1). Reliable and consistent quality is the basis for efficacy and safety of herbal medicinal products. Proper validation of herbs used in TM needs to be done for their promotion and development.
In conducting a clinical trial or during the preparation of food supplements, it is essential to clearly identify the plant component. The botanical identification is the first step of correct work. As a guidance tool, some experienced and tested approaches have been proposed to assist researchers in determining the quality and the safety of plant material (Zangara and Ghosh, 2014, “Clinical aspects of functional foods and nutraceuticals,” CRC Press).
Quality Issues
Excipients used in both pharmaceutical and nutraceuticals are no longer inert materials but they are effective and able to improve the characteristics of the products’ quality, stability, functionality, safety, solubility and acceptance among patients. They can change the medicament characteristics when they interact with the active ingredients (Abdellah A et al., 2015, Saudi Pharmaceutical Journal).
Since the introduction of the good manufacturing practice (GMP) regulations in the U.S. in 2007, the dietary supplements industry has continued to experience solid growth and now it’s part of the product approval system in many countries. Now the onus is on dietary supplement manufacturers/marketers to ensure that dietary supplements are safe and meet the quality standards that consumers and regulators are expecting. This can only be achieved through the rigorous application of in-house hazard analysis and critical control points (HACCP) and GMP systems from incoming raw material qualification and compliance to finished products (LeDoux et al., European Journal of Nutrition, 2015: 54).
The Way Forward
Several recent cases of adulteration and contamination of herbal medicinal products, which may have led to severe illness and possibly death in some cases, have revealed the necessity and urgency of identifying the plant material in botanicals and phytomedicines.
The older system of organoleptic (or identification through microscopic observation of plant parts) is not convincing, and plants are often misidentified. Recently, DNA-based methods have been applied to these products because “DNA is not changed by growth conditions unlike the chemical constituents of many active pharmaceutical agents,” (Moraes et al., 2015, Planta Medica).
The New York State attorney general recently called for the removal of certain herbal supplements from retail stores because DNA barcoding allegedly showed that most of the tested products did not contain the herbs listed on their labels. Although there is a strong counter-argument, DNA-based authentication of the ingredients incorporated into natural products is “perhaps the most efficient and economical method by which adverse effects of adulterants and plant or microbial contaminants may be minimized,” (Moraes et al 2015).
Dilip Ghosh, PhD, FACN
nutriConnect
Dilip Ghosh, PhD, FACN, is director of nutriConnect, based in Sydney, Australia. He is also professionally involved with Soho Flordis International, the University of Western Sydney, Australia, and is an Honorary Ambassador with the Global Harmonization Initiative (GHI). Dr. Ghosh received his PhD in biomedical science from University of Calcutta, India. He has been involved in drug-development (both synthetic and natural) and functional food research and development both in academic and industry domains. Dr. Ghosh has published more than 60 papers in peer-reviewed journals, and he has authored two recent books, “Biotechnology in Functional Foods and Nutraceuticals,” and “Innovation in Healthy and Functional Foods,” under CRC Press. His next book, “Clinical Perspective of Functional Foods and Nutraceuticals” is in press. He can be reached at dilipghosh@nutriconnect.com.au; www.nutriconnect.com.au