By Sean Moloughney, Editor09.26.18
In a viewpoint published in JAMA Internal Medicine titled “Probiotic Safety – No Guarantees,” Pieter Cohen, MD, Cambridge Health Alliance, Harvard Medical School, recently called into question the quality, safety, and efficacy of probiotics, claiming companies have capitalized on hype around the microbiome, spinning hundreds of small studies in their favor and engaging in “creative advertising.”
“For centuries, people have consumed live bacteria in many foods, such as yogurt, cheese, kimchi, and sauerkraut,” he wrote. “The mass-marketing of isolated live bacteria for their purported beneficial or ‘probiotic’ properties, however, is a relatively recent phenomenon. The World Health Organization defines probiotics as ‘live microorganisms which when administered in adequate amounts confer a health benefit on the host.’ Yet to be sold as a probiotic supplement in the United States, a live microorganism does not require evidence of efficacy or even safety.”
He went on to write that while research supports certain strains for specific conditions, “Most microorganisms used in the production of food, however, do not have proven health benefits, and their safety when sold as probiotic supplements has not been fully established.”
He noted that between 2002 and 2012, consumption of probiotic supplements in the U.S. has doubled, outpacing clinical research. “Despite the advertised indications, there are no large, long-term clinical trials proving that probiotics offer clinical benefits for people who are already healthy.”
Andrea Wong, PhD, vice president, scientific & regulatory affairs, Council for Responsible Nutrition (CRN), said Dr. Cohen seems to “gloss over” major systematic reviews that have shown probiotics to be beneficial in the treatment or prevention of specific conditions. “There’s a large body of evidence out there,” she added, indicating benefits for minor digestive issues as well.
Dr. Cohen also claimed the “inherent infective qualities” of live microorganisms found in probiotic supplements may pose risks to certain people, “especially among immune-compromised individuals.”
“He misrepresents the risk of probiotics,” Dr. Wong responded, saying at-risk populations should always speak to their doctor or healthcare practitioner before deciding to take a probiotic.
Dr. Cohen’s other major criticism related to overall quality. He cited FDA findings of good manufacturing practice (GMP) violations during inspections of dietary supplement facilities, noting: “Most commonly, companies had failed to establish the identity, purity, strength, and composition of their final product.”
Generally, companies have a responsibility to ensure the safety of their products, Dr. Wong said. For any novel strain companies want to introduce into the marketplace, they must submit a New Dietary Ingredient Notification (NDIN) to the FDA demonstrating the ingredient is safe under the specified conditions of use.
Despite Dr. Cohen’s criticism, what consumers believe may be most important to product sales. Tom Vierhile, innovation insights director for GlobalData, noted that 65% of Americans believe probiotics have a positive impact on health, according to a GlobalData Q1 2017 consumer survey. “For perspective, 72% said the same for almonds, a food which routinely ranks at the top of this list. By age, 45-54 year olds were most likely to say that probiotics have a positive impact on health, with 78% of those consumers saying so. The lowest support, by age, was 18-24 year olds where just 48% said probiotics would have a positive impact on health.”
Still, there were some areas of Dr. Cohen’s article that industry could agree with. For example, he recommended companies provide the number of live microorganisms on labels, which is also part of CRN’s best practice guidelines, and indicate the specific strains used in the product.
“It’s important to be transparent on the label, as we know different strains have different benefits,” and give consumers the most accurate information possible, said Dr. Wong.
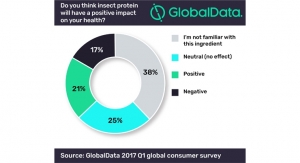
GlobalData also asked consumers about the specific claim “contains probiotics” and if they are interested in and buying products making this claim. According to GlobalData’s 2016 Q4 survey, 38% of Americans said they are interested in and are buying products that contain probiotics; 25-34 year olds showed the strongest interest, with 66% saying they are interested in and are buying products that “contain probiotics.” Interest tends to erode with age to the point where just 24% of 55-64 year olds said the same.
FDA Lableing Guidance
FDA recently issued a draft guidance, “Policy Regarding Quantitative Labeling of Dietary Supplements Containing Live Microbials,” in which the agency announced its intent to exercise enforcement discretion to allow supplement companies to use colony forming units (CFUs) when declaring the quantity of live microbials on a Supplement Facts Label.
“CFU is the scientifically accepted unit of measure for probiotics,” said Dr. Wong, and labeling live microbial dietary ingredients in CFUs gives consumers the best information possible when it comes to the viable microorganisms present in the product throughout shelf life. We are encouraged by FDA’s receptiveness to collaborate with industry to provide consumers with meaningful label information to inform their purchase decisions.”
However, the draft guidance states the label must also list the quantitative amount by weight, as is required by current regulation applicable generally to other dietary supplements, in addition to an expression of CFUs, and still requires listing in order of predominance by weight.
“To that extent, FDA’s guidance missed the mark,” said Dr. Wong. “CRN cautions that weight does not correlate with the number of viable microorganisms in a product. Therefore, it is not possible to accurately declare quantity in both weight and CFUs on a consistent basis. Listing the weight of probiotic contents does not provide consumers with useful information for comparing probiotic products and making buying decisions. A dual listing of ingredient quantity in weight and CFUs, as proposed by the Draft Guidance, presents conflicting product information on the label and puts responsible industry members in an untenable position.”
CRN has advocated for the acceptance of CFUs as the unit of measure for probiotics over the past several years and will continue to engage with FDA on this important issue, she added. “CRN intends to submit comments on the Draft Guidance and encourages industry members to voice their concerns as well.”
“For centuries, people have consumed live bacteria in many foods, such as yogurt, cheese, kimchi, and sauerkraut,” he wrote. “The mass-marketing of isolated live bacteria for their purported beneficial or ‘probiotic’ properties, however, is a relatively recent phenomenon. The World Health Organization defines probiotics as ‘live microorganisms which when administered in adequate amounts confer a health benefit on the host.’ Yet to be sold as a probiotic supplement in the United States, a live microorganism does not require evidence of efficacy or even safety.”
He went on to write that while research supports certain strains for specific conditions, “Most microorganisms used in the production of food, however, do not have proven health benefits, and their safety when sold as probiotic supplements has not been fully established.”
He noted that between 2002 and 2012, consumption of probiotic supplements in the U.S. has doubled, outpacing clinical research. “Despite the advertised indications, there are no large, long-term clinical trials proving that probiotics offer clinical benefits for people who are already healthy.”
Andrea Wong, PhD, vice president, scientific & regulatory affairs, Council for Responsible Nutrition (CRN), said Dr. Cohen seems to “gloss over” major systematic reviews that have shown probiotics to be beneficial in the treatment or prevention of specific conditions. “There’s a large body of evidence out there,” she added, indicating benefits for minor digestive issues as well.
Dr. Cohen also claimed the “inherent infective qualities” of live microorganisms found in probiotic supplements may pose risks to certain people, “especially among immune-compromised individuals.”
“He misrepresents the risk of probiotics,” Dr. Wong responded, saying at-risk populations should always speak to their doctor or healthcare practitioner before deciding to take a probiotic.
Dr. Cohen’s other major criticism related to overall quality. He cited FDA findings of good manufacturing practice (GMP) violations during inspections of dietary supplement facilities, noting: “Most commonly, companies had failed to establish the identity, purity, strength, and composition of their final product.”
Generally, companies have a responsibility to ensure the safety of their products, Dr. Wong said. For any novel strain companies want to introduce into the marketplace, they must submit a New Dietary Ingredient Notification (NDIN) to the FDA demonstrating the ingredient is safe under the specified conditions of use.
Despite Dr. Cohen’s criticism, what consumers believe may be most important to product sales. Tom Vierhile, innovation insights director for GlobalData, noted that 65% of Americans believe probiotics have a positive impact on health, according to a GlobalData Q1 2017 consumer survey. “For perspective, 72% said the same for almonds, a food which routinely ranks at the top of this list. By age, 45-54 year olds were most likely to say that probiotics have a positive impact on health, with 78% of those consumers saying so. The lowest support, by age, was 18-24 year olds where just 48% said probiotics would have a positive impact on health.”
Still, there were some areas of Dr. Cohen’s article that industry could agree with. For example, he recommended companies provide the number of live microorganisms on labels, which is also part of CRN’s best practice guidelines, and indicate the specific strains used in the product.
“It’s important to be transparent on the label, as we know different strains have different benefits,” and give consumers the most accurate information possible, said Dr. Wong.
GlobalData also asked consumers about the specific claim “contains probiotics” and if they are interested in and buying products making this claim. According to GlobalData’s 2016 Q4 survey, 38% of Americans said they are interested in and are buying products that contain probiotics; 25-34 year olds showed the strongest interest, with 66% saying they are interested in and are buying products that “contain probiotics.” Interest tends to erode with age to the point where just 24% of 55-64 year olds said the same.
FDA Lableing Guidance
FDA recently issued a draft guidance, “Policy Regarding Quantitative Labeling of Dietary Supplements Containing Live Microbials,” in which the agency announced its intent to exercise enforcement discretion to allow supplement companies to use colony forming units (CFUs) when declaring the quantity of live microbials on a Supplement Facts Label.
“CFU is the scientifically accepted unit of measure for probiotics,” said Dr. Wong, and labeling live microbial dietary ingredients in CFUs gives consumers the best information possible when it comes to the viable microorganisms present in the product throughout shelf life. We are encouraged by FDA’s receptiveness to collaborate with industry to provide consumers with meaningful label information to inform their purchase decisions.”
However, the draft guidance states the label must also list the quantitative amount by weight, as is required by current regulation applicable generally to other dietary supplements, in addition to an expression of CFUs, and still requires listing in order of predominance by weight.
“To that extent, FDA’s guidance missed the mark,” said Dr. Wong. “CRN cautions that weight does not correlate with the number of viable microorganisms in a product. Therefore, it is not possible to accurately declare quantity in both weight and CFUs on a consistent basis. Listing the weight of probiotic contents does not provide consumers with useful information for comparing probiotic products and making buying decisions. A dual listing of ingredient quantity in weight and CFUs, as proposed by the Draft Guidance, presents conflicting product information on the label and puts responsible industry members in an untenable position.”
CRN has advocated for the acceptance of CFUs as the unit of measure for probiotics over the past several years and will continue to engage with FDA on this important issue, she added. “CRN intends to submit comments on the Draft Guidance and encourages industry members to voice their concerns as well.”