Lisa Katic, RD10.30.15
Never has there been a safer or more tested technology used to develop new and improved agricultural crops than genetic engineering. Genetically engineered crops can withstand drought and other extreme climatic conditions, deliver greater yields and use fewer resources to grow more food.
Since these crops were first planted in the late 1990s, 18 million farmers in 28 countries have grown 448 million acres of genetically engineered crops and consumers have eaten billions of pounds of food harvested from these farms. In addition, an overwhelming majority of scientists from credible organizations, such as the American Association for the Advancement of Science, support the safety of these crops and foods.1 If a technology can do all this, and do it safely, then why all the fuss?
The fuss begins with improper use of names and terminology, which has misled the public and confused the discussion around this technology. Food biotechnology, genetic engineering or GMO (which stands for genetically modified organism) are all used to describe similar agricultural practices and technologies.
However, one of them is not technically accurate: GMO. There are no actual living organisms in a food or ingredient derived by genetic engineering. Genetic engineering is a process, not an organism, and it’s achieved by selecting beneficial or desired traits from one crop or species and inserting it into another. This process takes place within the seed so the plant will grow and contain the desired trait. This works just like traditional breeding, which has been practiced for thousands of years. The difference is that genetic engineering achieves this result faster with more precise results.
Genetics 101
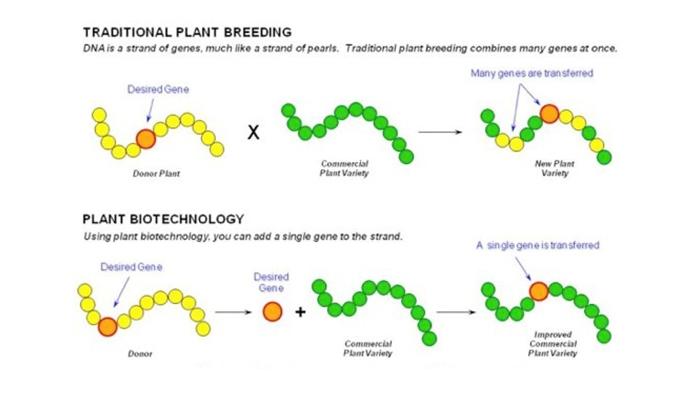
In order to explain genetic engineering, it is important to revisit the basics of genetics. Remember that all DNA are made up of genes, which determine the characteristics of the offspring. Think of a strand of DNA like a string of pearls. Imagine that you have a white string of pearls and a gray string of pearls; now visualize combining both stands to get a mixture of pearls (genes). The sequence and outcome is very random and not well known or controlled.
This is how traditional plant breeding has always been done. Using new techniques like genetic engineering, scientists can identify and move one desired gene and place it in the proper place along a DNA strand (string of pearls). This process is much more precise and the outcome is predictable, unlike with traditional breeding (see diagram below).
Now that you understand the mechanics of genetic modification, let’s review this technology’s evaluation of safety and regulatory structure.
Safety Assessment & Regulatory Framework
The U.S. regulatory framework for biotech foods is based on the Coordinated Framework for Regulation of Biotechnology Products, published in the federal register on June 26, 1986.2 The regulatory structure that exists for these foods is comprehensive and requires input from the United States Food and Drug Administration, United States Department of Agriculture and the Environmental Protection Agency. Before any genetically modified food can enter the food supply, it is tested by the developer of the product and reviewed for its nutritional properties, toxicity and allergenicity.
Since the coordinated framework for regulation of these crops is based on the notion that the modified crop is the same as its conventional counterpart in terms of function and expected properties, the following categories are used to determine if a bioengineered crop is deemed the same as its traditional counterpart:3
· The modified crop must be evaluated for toxicant characteristics of the host and donor species.
· Food allergens must not have been extracted and/or transferred from one source to another.
· Concentration and bioavailability of important nutrients must not be changed in the new product.
· Safety and nutritional value of newly introduced proteins must be well documented and no different than the conventional counterpart of food.
· The food must have been produced using accepted, established procedures.
Numerous scientific organizations, including the National Academy of Sciences, the American Medical Association and the World Health Organization have reviewed this technology and deemed it safe for the public. In addition, more than 1,700 studies have been conducted on genetically engineered crops over several decades, and found no evidence of harm. Access to these studies can found at: http://genera.biofortified.org.
Is There a Risk of Food Allergies?
There are no commercially available crops that introduced new allergens by using genetic engineering. The rigorous testing process ensures this is very unlikely to happen. There are thousands of proteins in the diet and only eight of them cause allergies. The USDA requires companies to analyze the proteins they use in the biotech process to determine if they are allergenic. This entire process is well regulated, monitored and can take up to 10 or more years to complete.
The only documented case where an actual allergen was introduced into a plant through genetic engineering occurred during an experiment that used a Brazil nut protein to improve the nutritional quality of a soybean.4 The Brazil nut protein that was isolated and carried the desired trait for this experiment’s purpose was an allergen. This prompted the researchers to halt the experiment, so the new soybean never entered the market. One aspect of the allergen discussion that is never mentioned is that we have existing foods in the food supply that cause significant allergies in some people. These foods were never put through the same scrutiny and testing procedures as products that are derived from genetic engineering.
How Can I Become More Educated About This Technology?
Food professionals and industry leaders need to be armed with the facts about agriculture and genetic engineering to effectively answer questions and inform others about the technology. To learn more about these issues, here are several credible and fact-based resources to consider:
· International Food Information Council (www.foodinsight.org).
· International Food Information Council Foundation, Food Biotechnology: A Communicators Guide to Improving Understanding, March 19, 2015 (www.foodinsight.org/education/food-biotechnology-communicator’s-guide-improving-understanding)
· International Food Information Council, Understanding and Communicating about Biotechnology; A CPE for Registered Dietitian Nutritionists, June 10, 2015 (www.foodinsight.org/continuing-education-cpe-credit-biotechnology-food)
· GMO Answers (www.gmoanswers.com)
· Soy Connection’s “PhiloSOYphy” page, brought to you by the United Soybean Board (/www.soyconnection.com/soy-wisdom)
· Consumer Science in the Public Interest – Straight Talk on Genetically Engineered Foods (http://cspinet.org/new/pdf/biotech-faq.pdf)
· Wayne Parrott Podcast on Biotech; Department of Crop & Soil Sciences and Institute for Plant Breeding, University of Georgia (www.talkingbiotechpodcast.com)
· Genetic Literacy Project (www.geneticliteracyproject.org)
· Institute of Food Technologists; What You Need to Know About GMO’s, GM Crops and the Techniques of Modern Biotechnology (http://sciencemeetsfood.org/need-know-gmos-gm-crops-techniques-modern-biotechnology/)
Lisa Katic is a registered dietitian and expert in food policy, biotechnology and food labeling and standards for international commerce. She has appeared on the Today Show, CNN and the Food Network, among others, to discuss current food policy issues.
References
1. Public and Scientists’ Views on Science and Society, Pew Research Center, January 29, 2015; /www.pewinternet.org/2015/01/29/public-and-scientists-views-on-science-and-society/.
2. Coordinated Framework for Regulation of Biotechnology, Executive Office of the President, Office of Science and Technology Policy, June 26, 1986; www.aphis.usda.gov/brs/fedregister/coordinated_framework.pdf
3. Statement of Policy - Foods Derived from New Plant Varieties, Guidance to Industry for Foods Derived from New Plant Varieties, U.S. Food and Drug Administration, May 29, 1992; /www.fda.gov/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/Biotechnology/ucm096095.htm.
4. Nordlee JA, Taylor SL, Townsend JA, Thomas LA and Bush RK (1996). Identification of a Brazil-nut allergen in transgenic soybeans. N. Engl. J. Med., 334:688–92. www.ncbi.nlm.nih.gov/pubmed/8594427
Since these crops were first planted in the late 1990s, 18 million farmers in 28 countries have grown 448 million acres of genetically engineered crops and consumers have eaten billions of pounds of food harvested from these farms. In addition, an overwhelming majority of scientists from credible organizations, such as the American Association for the Advancement of Science, support the safety of these crops and foods.1 If a technology can do all this, and do it safely, then why all the fuss?
The fuss begins with improper use of names and terminology, which has misled the public and confused the discussion around this technology. Food biotechnology, genetic engineering or GMO (which stands for genetically modified organism) are all used to describe similar agricultural practices and technologies.
However, one of them is not technically accurate: GMO. There are no actual living organisms in a food or ingredient derived by genetic engineering. Genetic engineering is a process, not an organism, and it’s achieved by selecting beneficial or desired traits from one crop or species and inserting it into another. This process takes place within the seed so the plant will grow and contain the desired trait. This works just like traditional breeding, which has been practiced for thousands of years. The difference is that genetic engineering achieves this result faster with more precise results.
Genetics 101
In order to explain genetic engineering, it is important to revisit the basics of genetics. Remember that all DNA are made up of genes, which determine the characteristics of the offspring. Think of a strand of DNA like a string of pearls. Imagine that you have a white string of pearls and a gray string of pearls; now visualize combining both stands to get a mixture of pearls (genes). The sequence and outcome is very random and not well known or controlled.
This is how traditional plant breeding has always been done. Using new techniques like genetic engineering, scientists can identify and move one desired gene and place it in the proper place along a DNA strand (string of pearls). This process is much more precise and the outcome is predictable, unlike with traditional breeding (see diagram below).
Now that you understand the mechanics of genetic modification, let’s review this technology’s evaluation of safety and regulatory structure.
Safety Assessment & Regulatory Framework
The U.S. regulatory framework for biotech foods is based on the Coordinated Framework for Regulation of Biotechnology Products, published in the federal register on June 26, 1986.2 The regulatory structure that exists for these foods is comprehensive and requires input from the United States Food and Drug Administration, United States Department of Agriculture and the Environmental Protection Agency. Before any genetically modified food can enter the food supply, it is tested by the developer of the product and reviewed for its nutritional properties, toxicity and allergenicity.
Since the coordinated framework for regulation of these crops is based on the notion that the modified crop is the same as its conventional counterpart in terms of function and expected properties, the following categories are used to determine if a bioengineered crop is deemed the same as its traditional counterpart:3
· The modified crop must be evaluated for toxicant characteristics of the host and donor species.
· Food allergens must not have been extracted and/or transferred from one source to another.
· Concentration and bioavailability of important nutrients must not be changed in the new product.
· Safety and nutritional value of newly introduced proteins must be well documented and no different than the conventional counterpart of food.
· The food must have been produced using accepted, established procedures.
Numerous scientific organizations, including the National Academy of Sciences, the American Medical Association and the World Health Organization have reviewed this technology and deemed it safe for the public. In addition, more than 1,700 studies have been conducted on genetically engineered crops over several decades, and found no evidence of harm. Access to these studies can found at: http://genera.biofortified.org.
Is There a Risk of Food Allergies?
There are no commercially available crops that introduced new allergens by using genetic engineering. The rigorous testing process ensures this is very unlikely to happen. There are thousands of proteins in the diet and only eight of them cause allergies. The USDA requires companies to analyze the proteins they use in the biotech process to determine if they are allergenic. This entire process is well regulated, monitored and can take up to 10 or more years to complete.
The only documented case where an actual allergen was introduced into a plant through genetic engineering occurred during an experiment that used a Brazil nut protein to improve the nutritional quality of a soybean.4 The Brazil nut protein that was isolated and carried the desired trait for this experiment’s purpose was an allergen. This prompted the researchers to halt the experiment, so the new soybean never entered the market. One aspect of the allergen discussion that is never mentioned is that we have existing foods in the food supply that cause significant allergies in some people. These foods were never put through the same scrutiny and testing procedures as products that are derived from genetic engineering.
How Can I Become More Educated About This Technology?
Food professionals and industry leaders need to be armed with the facts about agriculture and genetic engineering to effectively answer questions and inform others about the technology. To learn more about these issues, here are several credible and fact-based resources to consider:
· International Food Information Council (www.foodinsight.org).
· International Food Information Council Foundation, Food Biotechnology: A Communicators Guide to Improving Understanding, March 19, 2015 (www.foodinsight.org/education/food-biotechnology-communicator’s-guide-improving-understanding)
· International Food Information Council, Understanding and Communicating about Biotechnology; A CPE for Registered Dietitian Nutritionists, June 10, 2015 (www.foodinsight.org/continuing-education-cpe-credit-biotechnology-food)
· GMO Answers (www.gmoanswers.com)
· Soy Connection’s “PhiloSOYphy” page, brought to you by the United Soybean Board (/www.soyconnection.com/soy-wisdom)
· Consumer Science in the Public Interest – Straight Talk on Genetically Engineered Foods (http://cspinet.org/new/pdf/biotech-faq.pdf)
· Wayne Parrott Podcast on Biotech; Department of Crop & Soil Sciences and Institute for Plant Breeding, University of Georgia (www.talkingbiotechpodcast.com)
· Genetic Literacy Project (www.geneticliteracyproject.org)
· Institute of Food Technologists; What You Need to Know About GMO’s, GM Crops and the Techniques of Modern Biotechnology (http://sciencemeetsfood.org/need-know-gmos-gm-crops-techniques-modern-biotechnology/)
Lisa Katic is a registered dietitian and expert in food policy, biotechnology and food labeling and standards for international commerce. She has appeared on the Today Show, CNN and the Food Network, among others, to discuss current food policy issues.
References
1. Public and Scientists’ Views on Science and Society, Pew Research Center, January 29, 2015; /www.pewinternet.org/2015/01/29/public-and-scientists-views-on-science-and-society/.
2. Coordinated Framework for Regulation of Biotechnology, Executive Office of the President, Office of Science and Technology Policy, June 26, 1986; www.aphis.usda.gov/brs/fedregister/coordinated_framework.pdf
3. Statement of Policy - Foods Derived from New Plant Varieties, Guidance to Industry for Foods Derived from New Plant Varieties, U.S. Food and Drug Administration, May 29, 1992; /www.fda.gov/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/Biotechnology/ucm096095.htm.
4. Nordlee JA, Taylor SL, Townsend JA, Thomas LA and Bush RK (1996). Identification of a Brazil-nut allergen in transgenic soybeans. N. Engl. J. Med., 334:688–92. www.ncbi.nlm.nih.gov/pubmed/8594427