By Tania John, VP, NHP & Pharmaceutical Regulatory Sciences, Nutrasource Pharmaceutical and Nutraceutical Services04.13.23
Although cannabis use dates back centuries, it only became legal in Canada recreationally on Oct. 17, 2018, with edible cannabis products, extracts, and topicals permitted as of Oct. 17, 2019.1
Like any other plant, cannabis contains various chemical compounds. More than 100 of these substances are characterized as cannabinoids or phytocannabinoids.2 Cannabidiol (CBD) and delta-9-tetrahydrocannabinol (THC) are generally the best known and most popular phytocannabinoids, with the latter causing intoxication via its psychoactive properties.2
CBD on the other hand may even lessen some of THC’s effects when consumed together, though it still impacts the brain.2 For this reason, and taking regulatory restrictions into consideration, many formulators opt for low THC-CBD combinations or CBD-only offerings.
With the launch of the Cannabis Regulations, several dosage formats and routes of administration became available to CBD like eating or drinking edibles, ingesting and inhaling extracts, and applying topicals to the hair, skin, and nails.3

Only the parts of cannabis and hemp plants that do not meet the definition of cannabis under the Cannabis Act, or have been exempted by the Industrial Hemp Regulations, can be used in NHPs and VHPs (e.g., Cannabis sativa), as follows:
Such cannabis ingredients or parts cannot contain more than 10 ppm THC, or phytocannabinoids that have been isolated, concentrated, or deliberately added. Similarly, cosmetics can only contain hemp derivatives.2
As a reminder, recreational cannabis products cannot make health claims under the Cannabis Regulations. The Cannabis and Food and Drugs Acts do, however, allow for cannabis-containing prescription drugs and medical devices to be marketed and sold with health claims, provided that they are evidence-based, submitted for review and approved by Health Canada, and meet all criteria as outlined per the product category’s respective regulations.
This view is also expressed by a large portion of Generation Z that prefers cannabis alternatives over alcohol, arguably pushing CBD’s status from a trend to a mainstay staple. In general, consumers have greater health consciousness and unique needs that the cannabis market satisfies.
In order to meet this demand, suppliers, manufacturers, brands, and all relevant stakeholders must continue to conduct research and work with regulators to bring safe, effective, and innovative cannabis products to market.
 About the Author: Tania John, MSc, is Vice President, NHP and Pharmaceutical Regulatory Sciences for Nutrasource Pharmaceutical and Nutraceutical Services. She brings 15 years of regulatory and quality experience in the Canadian Natural Health Product (NHP) space, including cross-functional support and project management. She works closely with Nutrasource’s analytical, clinical, medical writing, business development, and marketing teams to ensure client objectives are exceeded through strong scientific substantiation and strategic partnership. She has a BSc and MSc from the University of Guelph, majoring in Nutrition & Nutraceutical Sciences and Food Safety & Quality Assurance, respectively. Prior to joining Nutrasource, John served as a Product Licensing & QA Associate at Now Foods/Puresource Inc. as Regulatory Affairs Specialist and, later, Associate Director, then Director of Regulatory Affairs. Recently, she became the Lead of the Global Prebiotic Association’s (GPA) Scientific & Technical Committee.
About the Author: Tania John, MSc, is Vice President, NHP and Pharmaceutical Regulatory Sciences for Nutrasource Pharmaceutical and Nutraceutical Services. She brings 15 years of regulatory and quality experience in the Canadian Natural Health Product (NHP) space, including cross-functional support and project management. She works closely with Nutrasource’s analytical, clinical, medical writing, business development, and marketing teams to ensure client objectives are exceeded through strong scientific substantiation and strategic partnership. She has a BSc and MSc from the University of Guelph, majoring in Nutrition & Nutraceutical Sciences and Food Safety & Quality Assurance, respectively. Prior to joining Nutrasource, John served as a Product Licensing & QA Associate at Now Foods/Puresource Inc. as Regulatory Affairs Specialist and, later, Associate Director, then Director of Regulatory Affairs. Recently, she became the Lead of the Global Prebiotic Association’s (GPA) Scientific & Technical Committee.
2. Health Canada. Cannabidiol (CBD), 2020. https://www.canada.ca/en/health-canada/services/drugs-medication/cannabis/about/cannabidiol.html
3. Health Canada. Final regulations: Edible cannabis, cannabis extracts, cannabis topicals, 2019. https://www.canada.ca/en/health-canada/services/drugs-medication/cannabis/resources/regulations-edible-cannabis-extracts-topicals.html
4. Health Canada. Cannabis Regulations (SOR/2018-144). https://laws-lois.justice.gc.ca/eng/regulations/sor-2018-144/
5. Health Canada. Natural Health Products Ingredients Database – Cannabis sativa, 2023. https://webprod.hc-sc.gc.ca/nhpid-bdipsn/ingredReq.do?id=5823&lang=eng
Like any other plant, cannabis contains various chemical compounds. More than 100 of these substances are characterized as cannabinoids or phytocannabinoids.2 Cannabidiol (CBD) and delta-9-tetrahydrocannabinol (THC) are generally the best known and most popular phytocannabinoids, with the latter causing intoxication via its psychoactive properties.2
CBD on the other hand may even lessen some of THC’s effects when consumed together, though it still impacts the brain.2 For this reason, and taking regulatory restrictions into consideration, many formulators opt for low THC-CBD combinations or CBD-only offerings.
With the launch of the Cannabis Regulations, several dosage formats and routes of administration became available to CBD like eating or drinking edibles, ingesting and inhaling extracts, and applying topicals to the hair, skin, and nails.3
Regulatory Landscape
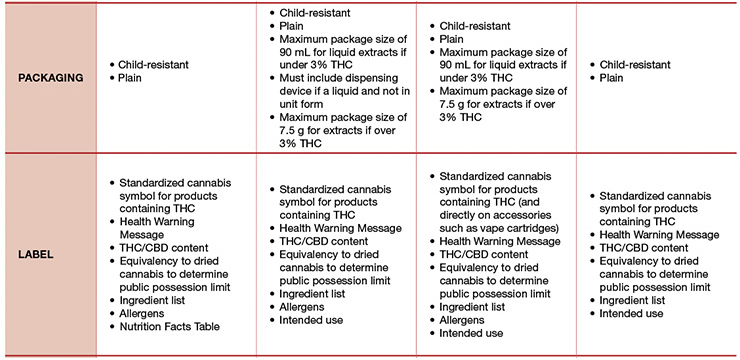
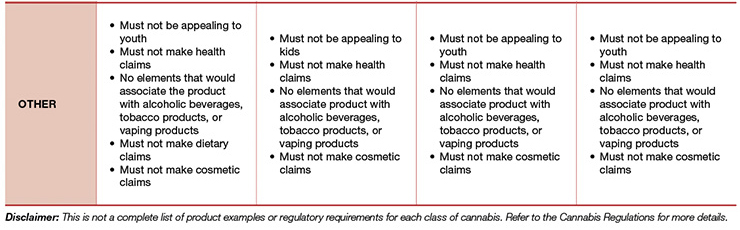
The implementation of any new regulatory framework naturally causes confusion. Questions around product rules; packaging, labeling, and claims; and other limitations are quite frequent and valid, especially in the beginning. The Final Regulations for Edible Cannabis, Cannabis Extracts, and Cannabis Topicals provides a helpful high-level overview, which has been adapted and included for reference in Table 1.3


Foods and Beverages
For products that adhere to the Cannabis Act, like edibles, Table 1 summarizes the key requirements across cannabis classes, however, common and/or notable themes are highlighted herein:- Edible cannabis products must be shelf-stable (i.e., they do not require freezing or refrigeration);
- Edible cannabis products cannot contain any ingredients other than food and food additives, and food additives must be used in accordance with the limits and purposes prescribed in the Food and Drug Regulations;
- Fortification with vitamins or mineral nutrients is not permitted;
- Use of alcohol is not permitted, with the exception of cannabis extracts that do not allow more than a 50% w/w concentration of ethyl alcohol;
- Use of caffeine as a food additive is not permitted, however, naturally occurring caffeine from chocolate, coffee, or tea, for example, is allowed provided that the total amount of caffeine in each container does not exceed 30 mg;
- Mixed products such as cannabis-infused alcoholic beverages or cannabis with tobacco or nicotine are prohibited;
- Cannabis products must not appeal to kids or youth; and
- Cannabis product cannot carry cosmetic, dietary, or health claims.3,4
Natural Health Products (NHPs)
The Cannabis Act (and associated Regulations) covers cannabis that is applied topically, inhaled, and consumed through foods, beverages, and even conventional supplement dosage formats like capsules. However, Natural Health Products (NHPs), as well as Veterinary Health Products (VHPs) and cosmetics, are outside of its purview.2,5Only the parts of cannabis and hemp plants that do not meet the definition of cannabis under the Cannabis Act, or have been exempted by the Industrial Hemp Regulations, can be used in NHPs and VHPs (e.g., Cannabis sativa), as follows:
- Non-viable seeds;
- Hemp derivatives that are compliant with the Industrial Hemp Regulations;
- Mature stalks without any leaves, flowers, seeds, or branches, and fiber derived from such stalks. However, these forms are not acceptable for use in VHPs.2,5
Such cannabis ingredients or parts cannot contain more than 10 ppm THC, or phytocannabinoids that have been isolated, concentrated, or deliberately added. Similarly, cosmetics can only contain hemp derivatives.2
Pharmaceuticals
With cannabis products and other regulated categories like NHPs sharing dosage formats, it may be difficult to distinguish between items or understand the distinction. Essentially, it comes down to the part of the plant used and the product’s recommended use or purpose.As a reminder, recreational cannabis products cannot make health claims under the Cannabis Regulations. The Cannabis and Food and Drugs Acts do, however, allow for cannabis-containing prescription drugs and medical devices to be marketed and sold with health claims, provided that they are evidence-based, submitted for review and approved by Health Canada, and meet all criteria as outlined per the product category’s respective regulations.
The Future
The Cannabis Regulations came into effect just prior to the discovery of COVID-19, propelling the initial cannabis boom and excitement into uncertain times. As a result of the pandemic and ongoing global and economic issues, individuals are more concerned about their health and wellbeing than ever before. Many people are seeking natural pain, sleep, and stress solutions while reducing alcohol consumption or eliminating it altogether.This view is also expressed by a large portion of Generation Z that prefers cannabis alternatives over alcohol, arguably pushing CBD’s status from a trend to a mainstay staple. In general, consumers have greater health consciousness and unique needs that the cannabis market satisfies.
In order to meet this demand, suppliers, manufacturers, brands, and all relevant stakeholders must continue to conduct research and work with regulators to bring safe, effective, and innovative cannabis products to market.

References
1. Health Canada. Taking stock of progress: Cannabis legalization and regulation in Canada, 2022. https://www.canada.ca/en/health-canada/programs/engaging-cannabis-legalization-regulation-canada-taking-stock-progress/document.html#a132. Health Canada. Cannabidiol (CBD), 2020. https://www.canada.ca/en/health-canada/services/drugs-medication/cannabis/about/cannabidiol.html
3. Health Canada. Final regulations: Edible cannabis, cannabis extracts, cannabis topicals, 2019. https://www.canada.ca/en/health-canada/services/drugs-medication/cannabis/resources/regulations-edible-cannabis-extracts-topicals.html
4. Health Canada. Cannabis Regulations (SOR/2018-144). https://laws-lois.justice.gc.ca/eng/regulations/sor-2018-144/
5. Health Canada. Natural Health Products Ingredients Database – Cannabis sativa, 2023. https://webprod.hc-sc.gc.ca/nhpid-bdipsn/ingredReq.do?id=5823&lang=eng