By Joerg Gruenwald, analyze & realize ag11.01.18
The Nutrition and Health Claims Regulation (NHCR) EC 1924/2006 went into effect on July 1, 2007. Originally intended to end the potential misuse of unregulated health claims on food product labels and to bring clarity for the consumer, it also has, arguably, led to a stagnation of innovation in many categories, and even negative growth in some.
With the resulting strict regulation of health claims, the implementation of the NHCR has had a tremendous impact on how food supplements in particular are marketed. Over the years, a lot of the claims that were submitted for innovative ingredients (acc. to Art. 13.5 of the Health Claim regulation) received a negative opinion from the European Food Safety Authority (EFSA). In fact, the overall number of refused claim applications is high, as shown in Table 1.
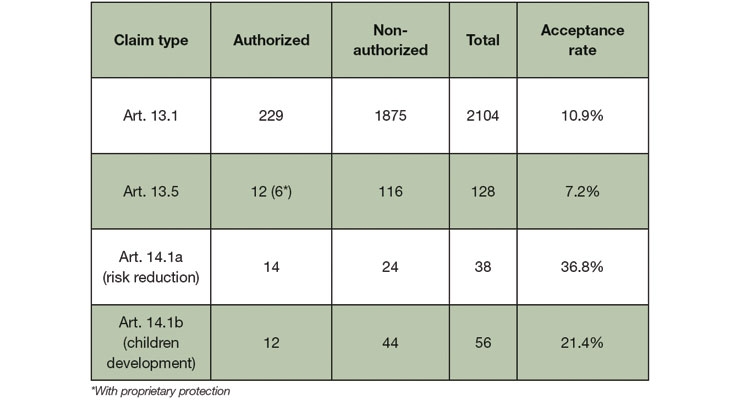
Table 1. Acceptance rates of health claims based on the EU Register of Nutrition and Health Claims
In the beginning, the low acceptance rate could be explained in part by the fact that applicants went on the assumption that claims made on food products would not require a drug-level effect substantiation approach, and they tailored their applications accordingly. As they soon learned, they could hardly have been more wrong.
It seems that EFSA also underwent (and is still undergoing) a learning curve. The process eventually resulted in the publication of various guidance papers intended to assist applicants in designing an EFSA-conforming trial and reporting the study results in an appropriate way.
Indeed, it was these guidance papers that shed light on the requirements EFSA has for the substantiation of a health claim. Also, the sheer number of published opinions further clarifies the way EFSA evaluates applications. Nevertheless, the scientific hurdles are still high as EFSA insists on high-quality studies and excellent reporting.
A Clearer Picture: A Better Result
With the approval process becoming clearer, applying for a health claim is more worthwhile now than it was a few years ago. The reason is simply that authorized (generic) health claims as published in the EU Register of nutrition and health claims do not allow for much product differentiation. Also, B2B companies that can offer an authorized health claim for an innovative product to B2C companies have a definite competitive edge.
For food supplements, too, marketing without a health claim is still challenging, since these products cannot fall back on soft claims such as “great taste” or the kind of wellness or emotional claims the general food industry likes to resort to. Food supplements are still taken for health reasons, not for indulgence, which will continue to pose a challenge to the industry.
With the new Novel Food Regulation (NFR), it is now possible, according to Article 28 of the regulation, to synchronize applications for a novel food authorization and for a health claim so as not to infringe upon the 5-year protection period granted by the health claim application while the result of the novel food application is pending. In this way, the period of protection for the proprietary health claim will only start after the novel food authorization for the substance has been granted.
This might be of importance for food business operators currently working on a novel food application for an innovative ingredient, especially considering the different processing times involved compared to a health claim application with EFSA.
With these learnings in mind, industry might again consider investing time and money into health claim applications, ideally ones that come with proprietary protection. Experienced consultancies such as analyze & realize GmbH will be glad to assist.
Joerg Gruenwald
analyze & realize ag
Dr. Joerg Gruenwald is co-founder of analyze & realize GmbH, a specialized business consulting company and CRO in the fields of nutraceuticals, dietary supplements, herbals and functional food, and author of the PDR for Herbal Medicines. He can be reached at analyze & realize GmbH, Waldseeweg 6, 13467 Berlin, Germany; +49-30-40008100; E-mail: jgruenwald@a-r.com;
Website: www.analyze-realize.com.
With the resulting strict regulation of health claims, the implementation of the NHCR has had a tremendous impact on how food supplements in particular are marketed. Over the years, a lot of the claims that were submitted for innovative ingredients (acc. to Art. 13.5 of the Health Claim regulation) received a negative opinion from the European Food Safety Authority (EFSA). In fact, the overall number of refused claim applications is high, as shown in Table 1.

Table 1. Acceptance rates of health claims based on the EU Register of Nutrition and Health Claims
In the beginning, the low acceptance rate could be explained in part by the fact that applicants went on the assumption that claims made on food products would not require a drug-level effect substantiation approach, and they tailored their applications accordingly. As they soon learned, they could hardly have been more wrong.
It seems that EFSA also underwent (and is still undergoing) a learning curve. The process eventually resulted in the publication of various guidance papers intended to assist applicants in designing an EFSA-conforming trial and reporting the study results in an appropriate way.
Indeed, it was these guidance papers that shed light on the requirements EFSA has for the substantiation of a health claim. Also, the sheer number of published opinions further clarifies the way EFSA evaluates applications. Nevertheless, the scientific hurdles are still high as EFSA insists on high-quality studies and excellent reporting.
A Clearer Picture: A Better Result
With the approval process becoming clearer, applying for a health claim is more worthwhile now than it was a few years ago. The reason is simply that authorized (generic) health claims as published in the EU Register of nutrition and health claims do not allow for much product differentiation. Also, B2B companies that can offer an authorized health claim for an innovative product to B2C companies have a definite competitive edge.
For food supplements, too, marketing without a health claim is still challenging, since these products cannot fall back on soft claims such as “great taste” or the kind of wellness or emotional claims the general food industry likes to resort to. Food supplements are still taken for health reasons, not for indulgence, which will continue to pose a challenge to the industry.
With the new Novel Food Regulation (NFR), it is now possible, according to Article 28 of the regulation, to synchronize applications for a novel food authorization and for a health claim so as not to infringe upon the 5-year protection period granted by the health claim application while the result of the novel food application is pending. In this way, the period of protection for the proprietary health claim will only start after the novel food authorization for the substance has been granted.
This might be of importance for food business operators currently working on a novel food application for an innovative ingredient, especially considering the different processing times involved compared to a health claim application with EFSA.
With these learnings in mind, industry might again consider investing time and money into health claim applications, ideally ones that come with proprietary protection. Experienced consultancies such as analyze & realize GmbH will be glad to assist.
Joerg Gruenwald
analyze & realize ag
Dr. Joerg Gruenwald is co-founder of analyze & realize GmbH, a specialized business consulting company and CRO in the fields of nutraceuticals, dietary supplements, herbals and functional food, and author of the PDR for Herbal Medicines. He can be reached at analyze & realize GmbH, Waldseeweg 6, 13467 Berlin, Germany; +49-30-40008100; E-mail: jgruenwald@a-r.com;
Website: www.analyze-realize.com.