04.20.23
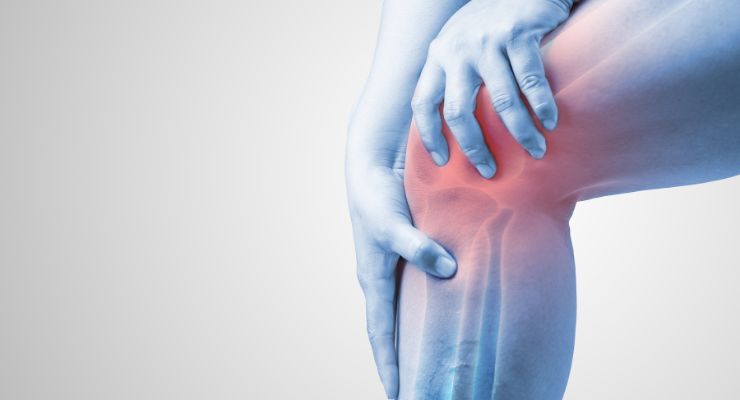
Valensa International's FlexPro MD, a blend of omega-3 fatty acids, Valensa’s Zanthin astaxanthin ingredient, and Valensa’s Flexonic hyaluronic acid, was recently awarded functional ingredient certification for Functional Health Foods in the Republic of Korea. The ingredient is formulated for joint health.
According to the Ministry of Food and Drug Safety MFDS in Korea, FlexPro MD is approved in compliance with the Functional Foods Act, and products carrying the ingredient can make functional claims related to healthy joint maintenance.
The certification was based on the strength of two mechanistic studies and two human clinical trials. Korean functional ingredient certification for the ingredient was a culmination of a multi-year project to design and conduct the clinical trials, manufacture trial materials, and test the active ingredients using standard methods.
The formula and each of the ingredients contained within were scrutinized by the MFDS and Novarex Co., Ltd. for safety and efficacy. The company partnered with Novarex for this registration process.
In one mechanistic study, the formula was shown to significantly inhibit NF-κB activation, expression levels of pro-inflammatory markers such as, TNF-α, IL-6 and IL 1β and matrix-degradation enzymes MMP1 and MMP2. In a second preclinical study on animal models, the ingredient was linked to reduced pain behavior and decreased articular cartilage destruction.
The first human clinical study, conducted by Valensa in the U.S., provided evidence that FlexPro MD outperformed a standard full dose of glucosamine and chondroitin for providing joint comfort. A 12-week, multi-center human clinical study with 100 participants was also conducted to support the application.
The latter study showed a significant improvement in self-reported and physician-assessed joint pain measures, compared to placebo. The study found significant differences in joint pain, stiffness, and function. Some study participants experienced a joint discomfort reduction of greater than 50% over 12 weeks. The study is anticipated to be published later this year.
“FlexPro MD covers the critical factors of joint health care that consumers seek,” said Umasudan Pal, CEO of Valensa. “These factors include mediating joint discomfort, managing inflammatory cascades, improving joint stiffness and overall joint physical function. The product delivery form, small dose and effectiveness also improves compliance and customer repeat purchases. FlexPro MD formulation is available for discerning brands that wants to differentiate their joint health portfolio with a clinically studied formula that can also be customized for their customer segments. Valensa is evaluating licensees in specific channels and markets.”
According to the Ministry of Food and Drug Safety MFDS in Korea, FlexPro MD is approved in compliance with the Functional Foods Act, and products carrying the ingredient can make functional claims related to healthy joint maintenance.
The certification was based on the strength of two mechanistic studies and two human clinical trials. Korean functional ingredient certification for the ingredient was a culmination of a multi-year project to design and conduct the clinical trials, manufacture trial materials, and test the active ingredients using standard methods.
The formula and each of the ingredients contained within were scrutinized by the MFDS and Novarex Co., Ltd. for safety and efficacy. The company partnered with Novarex for this registration process.
In one mechanistic study, the formula was shown to significantly inhibit NF-κB activation, expression levels of pro-inflammatory markers such as, TNF-α, IL-6 and IL 1β and matrix-degradation enzymes MMP1 and MMP2. In a second preclinical study on animal models, the ingredient was linked to reduced pain behavior and decreased articular cartilage destruction.
The first human clinical study, conducted by Valensa in the U.S., provided evidence that FlexPro MD outperformed a standard full dose of glucosamine and chondroitin for providing joint comfort. A 12-week, multi-center human clinical study with 100 participants was also conducted to support the application.
The latter study showed a significant improvement in self-reported and physician-assessed joint pain measures, compared to placebo. The study found significant differences in joint pain, stiffness, and function. Some study participants experienced a joint discomfort reduction of greater than 50% over 12 weeks. The study is anticipated to be published later this year.
“FlexPro MD covers the critical factors of joint health care that consumers seek,” said Umasudan Pal, CEO of Valensa. “These factors include mediating joint discomfort, managing inflammatory cascades, improving joint stiffness and overall joint physical function. The product delivery form, small dose and effectiveness also improves compliance and customer repeat purchases. FlexPro MD formulation is available for discerning brands that wants to differentiate their joint health portfolio with a clinically studied formula that can also be customized for their customer segments. Valensa is evaluating licensees in specific channels and markets.”