By Michael McGuffin, President, American Herbal Products Association (AHPA)06.02.22
Critics of dietary supplements have argued that this class of goods is insufficiently regulated—calling dietary supplements everything from underregulated to flat out unregulated. They have used these false and misleading arguments to call for unnecessary new legislation. However, the U.S. Food and Drug Administration (FDA) already has sufficient authority to fully regulate dietary supplements. In fact, under federal law, dietary supplements are regulated at least as or more strictly than other food categories.
Under applicable federal regulations, manufacturers and marketers of dietary supplements are required to, for example:
Dietary supplement regulations differ from those that govern drugs and conventional foods when it comes to recalls and adverse event reporting, respectively.
Manufacturers and marketers of drugs and dietary supplements are required to submit serious adverse event reports (SAERs) to FDA, but there is no such requirement for conventional foods. FDA has mandatory recall authority for foods (including dietary supplements) but not for drugs. Adverse event reports and information reported by firms conducting voluntary recalls aid FDA in monitoring the safety of the various products it regulates. The data on both support the safety and sufficient regulation of dietary supplements.
Unlike with marketers of conventional foods, who have no enforceable obligation to report SAERS to FDA, marketers of dietary supplements must report SAERs to the agency because industry groups lobbied for the establishment of such requirements.
Beginning in 2002, the American Herbal Products Association (AHPA) began advocating for the establishment of an SAER law for dietary supplements. Over four years, AHPA coordinated and led industry support to establish a mandatory system for SAERs for dietary supplements through efforts such as: communicating directly with the U.S. Department of Health and Human Services and FDA; filing a citizen petition with FDA; and providing testimony to the U.S. Senate. These efforts culminated in the enactment of the Dietary Supplement and Nonprescription Consumer Protection Act of 2006, under which dietary supplement manufacturers must submit SAERs to FDA.
Data on SAERs submitted to FDA are available to the public through the Center for Food Safety and Applied Nutrition (CFSAN) Adverse Event Reporting System (CAERS). From 2018-2020, 53% of the adverse event reports listed in CAERS are for cosmetics, 32% are for dietary supplements, and 15% are for conventional foods (Figure 1). As with conventional foods, cosmetics are not subject to mandatory adverse event reporting requirements, yet cosmetics make up more than half of adverse events reports captured in this recent dataset from CAERs.
Figure 1.
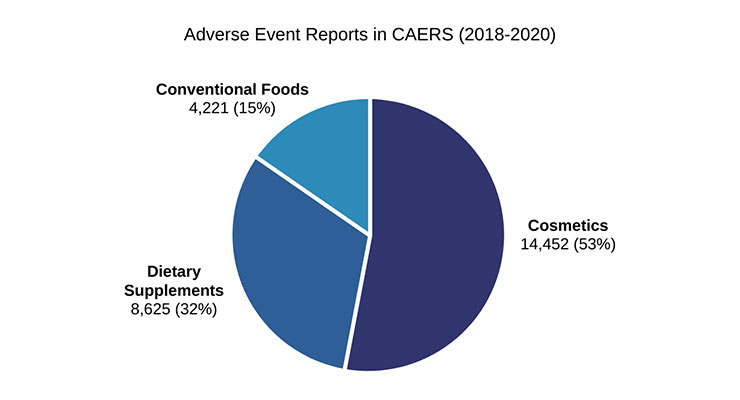
CAERS is one of the tools FDA uses to monitor the market for safety concerns. When FDA identifies a public health concern through adverse event reporting, the agency can take administrative or enforcement action to protect the public health. This can include requesting a recall or, in the case of foods (including dietary supplements), actually ordering one.
FDA has the authority to mandate a recall of foods (including dietary supplements) when the agency determines there is a reasonable probability that the food is adulterated (or misbranded due to a violation of major food allergen labeling requirements) and that the use of or exposure to the food will cause serious adverse health consequences or death to humans or animals.
However, the agency can only request a recall of drugs, even if a drug has been identified as defective or potentially harmful. Recalls for conventional foods—such as produce (due to contamination) or processed food (due to undeclared allergens)—are most common.
Of the nearly 2,000 recalls from 2019-2022 listed on the FDA website, only 3% of recalls were for dietary supplements, compared to 63% for conventional food and beverages, and 21% for drugs in that same time period (Figure 2). FDA gathers this data from information provided by recalling firms as well as press releases and other public notices about certain recalls of FDA-regulated products.
Figure 2.

With fewer recalls than conventional foods and beverages, drugs, animal and veterinary products, and medical devices, dietary supplements could arguably be considered the safest class of goods regulated by FDA.
This argument could find further support in the mandatory reporting of SAERs to FDA for dietary supplements, which provides the agency with data to monitor the market for safety concerns, and FDA’s mandatory recall authority for dietary supplements, with which the agency can mandate removal of unsafe products.
Altogether, dietary supplement regulations are not only robust, but, in most cases, are as strict or stricter than those that govern conventional foods. From requirements for submission of SAERs to FDA and the agency’s mandatory recall authority, dietary supplement regulations have ensured access to, and the safety of, these products for our families, friends, and neighbors who choose dietary supplements to support their personal health.
About the Author: Michael McGuffin has served as president of the American Herbal Products Association since 1999, leading the association in its mission to promote the responsible and sustainable commerce of herbal products to ensure that consumers have informed access to a wide variety of safe herbal goods. McGuffin also serves on the boards of directors of the American Herbal Pharmacopoeia and United Plant Savers, and on the advisory boards of the USC School of Pharmacy regulatory science master’s degree program and the Appalachian Beginning Forest Farmers Coalition. An industry veteran, McGuffin has been active in the herbal community since 1974, and has taken the lead on legislative matters and regulatory advocacy that have shaped the herbal and natural products industry over the last several decades. For more information: www.ahpa.org.
Under applicable federal regulations, manufacturers and marketers of dietary supplements are required to, for example:
- Register their facilities with FDA;
- Adhere to current Good Manufacturing Practice (cGMP) regulations;
- Include nutrition information, an ingredient list, the manufacturer, packer, or distributor’s name and address, and contact information for receipt of adverse event information on their product labels;
- Include information on major food allergens on their labels;
- Comply with requirements to disclose bioengineered ingredients on their labels;
- Obtain premarket FDA authorization for certain product claims;
- Notify FDA of certain other product claims; and
- Include disclaimers on their labels for certain product claims.
Dietary supplement regulations differ from those that govern drugs and conventional foods when it comes to recalls and adverse event reporting, respectively.
Manufacturers and marketers of drugs and dietary supplements are required to submit serious adverse event reports (SAERs) to FDA, but there is no such requirement for conventional foods. FDA has mandatory recall authority for foods (including dietary supplements) but not for drugs. Adverse event reports and information reported by firms conducting voluntary recalls aid FDA in monitoring the safety of the various products it regulates. The data on both support the safety and sufficient regulation of dietary supplements.
Adverse Event Reports
Irrespective of the regulated category, the Federal Food, Drug, and Cosmetic Act (FD&C Act) defines an “adverse event” for over-the-counter drugs, prescription drugs, and dietary supplements as “any health-related event associated with the use of a [over-the-counter drug/prescription drug/dietary supplement] that is adverse.” Further, a “serious adverse event” is an adverse event that results in death or serious injury.Unlike with marketers of conventional foods, who have no enforceable obligation to report SAERS to FDA, marketers of dietary supplements must report SAERs to the agency because industry groups lobbied for the establishment of such requirements.
Beginning in 2002, the American Herbal Products Association (AHPA) began advocating for the establishment of an SAER law for dietary supplements. Over four years, AHPA coordinated and led industry support to establish a mandatory system for SAERs for dietary supplements through efforts such as: communicating directly with the U.S. Department of Health and Human Services and FDA; filing a citizen petition with FDA; and providing testimony to the U.S. Senate. These efforts culminated in the enactment of the Dietary Supplement and Nonprescription Consumer Protection Act of 2006, under which dietary supplement manufacturers must submit SAERs to FDA.
Data on SAERs submitted to FDA are available to the public through the Center for Food Safety and Applied Nutrition (CFSAN) Adverse Event Reporting System (CAERS). From 2018-2020, 53% of the adverse event reports listed in CAERS are for cosmetics, 32% are for dietary supplements, and 15% are for conventional foods (Figure 1). As with conventional foods, cosmetics are not subject to mandatory adverse event reporting requirements, yet cosmetics make up more than half of adverse events reports captured in this recent dataset from CAERs.
Figure 1.

CAERS is one of the tools FDA uses to monitor the market for safety concerns. When FDA identifies a public health concern through adverse event reporting, the agency can take administrative or enforcement action to protect the public health. This can include requesting a recall or, in the case of foods (including dietary supplements), actually ordering one.
Recalls
FDA defines a recall as “a firm’s removal or correction of a marketed product that the FDA considers to be in violation of the laws it administers and against which the agency would initiate legal action.” Recalls to correct or remove unsafe products from the market are not unique to the conventional food, dietary supplement, or drug industries. In fact, most consumer product categories in the U.S.—including everything from cars to toys—are subject to recall if they pose a danger to public health.FDA has the authority to mandate a recall of foods (including dietary supplements) when the agency determines there is a reasonable probability that the food is adulterated (or misbranded due to a violation of major food allergen labeling requirements) and that the use of or exposure to the food will cause serious adverse health consequences or death to humans or animals.
However, the agency can only request a recall of drugs, even if a drug has been identified as defective or potentially harmful. Recalls for conventional foods—such as produce (due to contamination) or processed food (due to undeclared allergens)—are most common.
Of the nearly 2,000 recalls from 2019-2022 listed on the FDA website, only 3% of recalls were for dietary supplements, compared to 63% for conventional food and beverages, and 21% for drugs in that same time period (Figure 2). FDA gathers this data from information provided by recalling firms as well as press releases and other public notices about certain recalls of FDA-regulated products.
Figure 2.

With fewer recalls than conventional foods and beverages, drugs, animal and veterinary products, and medical devices, dietary supplements could arguably be considered the safest class of goods regulated by FDA.
This argument could find further support in the mandatory reporting of SAERs to FDA for dietary supplements, which provides the agency with data to monitor the market for safety concerns, and FDA’s mandatory recall authority for dietary supplements, with which the agency can mandate removal of unsafe products.
Robustly Regulated & Balanced
In addition to FDA regulations, dietary supplements are also subject to:- Federal Trade Commission regulations for advertising (i.e., claims must be truthful and not misleading);
- U.S. Fish & Wildlife regulations for botanicals protected by the Convention on International Trade in Endangered Species of Wild Fauna and Flora; and
- State and local municipality regulations.
Altogether, dietary supplement regulations are not only robust, but, in most cases, are as strict or stricter than those that govern conventional foods. From requirements for submission of SAERs to FDA and the agency’s mandatory recall authority, dietary supplement regulations have ensured access to, and the safety of, these products for our families, friends, and neighbors who choose dietary supplements to support their personal health.
About the Author: Michael McGuffin has served as president of the American Herbal Products Association since 1999, leading the association in its mission to promote the responsible and sustainable commerce of herbal products to ensure that consumers have informed access to a wide variety of safe herbal goods. McGuffin also serves on the boards of directors of the American Herbal Pharmacopoeia and United Plant Savers, and on the advisory boards of the USC School of Pharmacy regulatory science master’s degree program and the Appalachian Beginning Forest Farmers Coalition. An industry veteran, McGuffin has been active in the herbal community since 1974, and has taken the lead on legislative matters and regulatory advocacy that have shaped the herbal and natural products industry over the last several decades. For more information: www.ahpa.org.




























