Predictions for the global cannabis market is expected to reach $57 billion by 20271. With growing demand, comes confusion around cannabis, cannabidiol (CBD) and hemp. It reached a tipping point when the 2018 Farm Bill federally legalized the production of industrial hemp-derived CBD containing less than .03 percent of THC (tetrahydrocannabinol). Most states though have not been able to update their laws to match this new federal law, leaving them without answers to CBD’s legalities.
To help sort through the confusion, the FDA recently scheduled a public hearing for CBD. However, we may not have answers to the legalities and claims of CBD for quite some time. This leaves individual companies deciding for themselves if or how to handle the manufacturing, labeling, marketing claims, and sale of CBD. Even with the legal hurdles, many brands might continue creating CBD products, while some might wait it out, and others will proactively look for legal alternatives that produce similar health benefits.
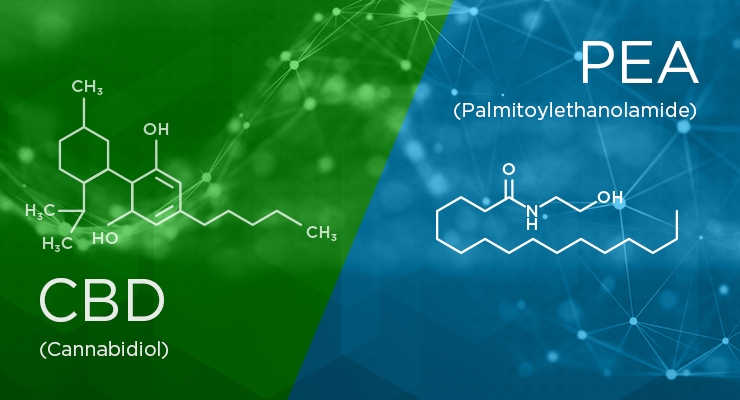
The Story of PEA versus CBD
Meet Palmitoylethanolamide or PEA, a legal alternative to CBD. PEA works within the Endocannabinoid System or ECS. ECS helps to maintain balance within our bodies and helps with regulating sleep, immune-system responses, relaxation, pain and more2. Our ECS naturally produces cannabinoids called endocannabinoids. CBD appears to inhibit the enzyme Fatty Acid Amide Hydrolase (FAAH), which breaks down anandamide (AEA), an endocannabinoid associated with regulating pain.
What makes PEA unique is its structural similarities to AEA. With PEA, studies have shown it may co-enhance the effects of AEA as well as inhibit FAAH. PEA also counteracts endotoxin-induced inflammation in cells in the same cell lineage as CBD. PEA and CBD share a number of centrally mediated effects which include neuroprotection and attenuation of seizure activity.
What’s the Catch?
PEA is naturally produced within the body as a direct response and repair mechanism to inflammation and pain. It is also a natural food component which can be isolated from soybeans, peanuts and egg yolks.
There are no known adverse effects of PEA supplementation. However, Gencor, a leader in clinically researched ingredients, discovered that PEA has a hard time dispersing in watery environments such as the stomach. To combat this, Gencor formulated its PEA, called Levagen+ PEA, with award-winning LipiSperse technology, which allows the PEA particles to disperse freely, translating into increased bioavailability.
Do Clinical Trials Back PEA?
Unlike CBD, PEA is well-researched. More than 21 clinical studies have shown the effectiveness of PEA as a safe nutrient for easing discomfort and aiding with a healthy inflammatory response. In addition to these studies, Gencor has conducted studies on the benefits of its branded PEA (Levagen) for joint health, sleep, and recovery.
Gencor’s human clinical trials indicated that Levagen PEA had significant positive effects on the total WOMAC pain score, joint stiffness and function as well as aids with post-exercise recovery. Additional clinical studies are in process to further back the efficacy of the ingredient.
In Conclusion
Levagen PEA has GRAS status and is clinically shown to be an effective CBD alternative for health benefits such as joint health, sleep, and recovery, making it a strong addition to any sports nutrition and healthy aging formulations.
To learn more visit GencorPacific.com
References:
- Badaracco, Suzy. “CBD Market and Latest Research.” Natural Products INSIDER, 9 Apr. 2019, www.naturalproductsinsider.com/herbs-botanicals/cbd-market-and-latest-research.
- Johnson, Jon. “CBD Oil for Pain Management: Effects, Benefits, and Uses.” Medical News Today, MediLexicon International, 16 Mar. 2018, www.medicalnewstoday.com/articles/319475.php.