10.30.18
Marking the 10th year of the Food and Drug Administration’s (FDA) GMP program, the Natural Products Association (NPA) pledged to continue working collaboratively with its members and government regulators to develop smart regulations that lead to strong government enforcement and better consumer protection.
“The U.S. has the safest nutritional supplements in the world because of collaborative efforts between our industry and federal regulators,” said Daniel Fabricant, PhD, president and CEO of NPA. “We are proud to have played a leading role developing these standards a decade ago and pledge to continue building upon this good work in the years to come.”
NPA has always taken a leadership role in promoting quality standards and has developed proactive certification programs for that purpose. NPA was the first organization to offer a third-party GMP certification program for the manufacturing of dietary supplements and dietary ingredients. NPA also maintains a warning letter database for NPA members to view violations of GMPs.
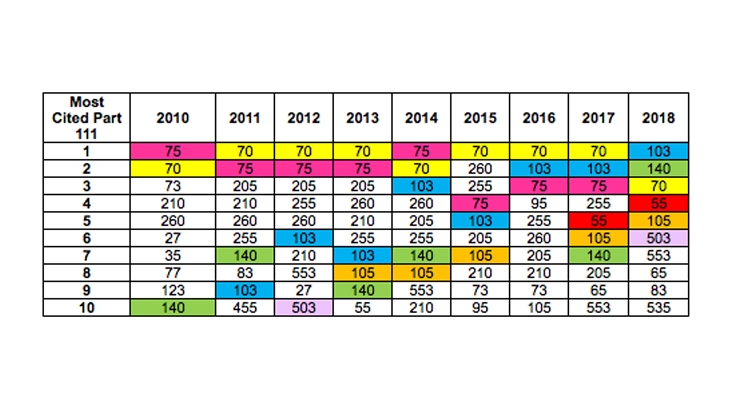
Most Cited Part 111 Charge as a Function of Year
The chart below includes the top 10 most cited Part 111 citations issued for each year, with the top 5 citations for 2018 color coded for year-over-year comparison. Notable trends based on NPA’s analysis of the data include:
Code Definitions:
27 Requirements that apply to equipment and utensils
35 Recordkeeping of equipment and utensils
55 Requirements to implement a production and process control system
65 Requirements for quality control operations when establishing a production and process control system
70 Specifications to establish when establishing a production and process control system
73 Responsibility for determining whether established specifications are met for establishing a production and process control system
75 Determining if specifications are met for establishing a production and process control system
77 Protocol for unmet established specifications for production and process control systems
83 Requirements for reserve samples when establishing a production and process control system
95 Recordkeeping when establishing a production & process control system
103 Written procedures for production and process control systems
105 Quality control personnel responsibilities for production and process control systems
123 Quality control operations that are required for the master manufacturing record, the batch production record, and manufacturing operations
140 Recordkeeping requirements for production and process control systems
205 Requirement to establish a master manufacturing record for each formulation of a dietary supplement
210 Requirements for the Master Manufacturing Record: What to include in master manufacturing record
255 Requirement to establish a batch production record
260 Production and Process Control System: Requirements for the Batch Production Record - Batch record inclusion requirements
455 Requirements that apply to holding components, dietary supplements, packaging, and labels
503 Requirements for written procedures for dietary supplements
535 Recordkeeping for returned dietary supplements
553 Written procedures for product complaints
NPA Educational Webinar
On October 30, 2018 NPA will host FDA officials and industry experts for an educational webinar examining the current trends in GMP compliance and enforcement for dietary supplements. Participants can register online by October 30, 2018. For more information about the webinar, please contact dnissinoff@npanational.org.
“The U.S. has the safest nutritional supplements in the world because of collaborative efforts between our industry and federal regulators,” said Daniel Fabricant, PhD, president and CEO of NPA. “We are proud to have played a leading role developing these standards a decade ago and pledge to continue building upon this good work in the years to come.”
NPA has always taken a leadership role in promoting quality standards and has developed proactive certification programs for that purpose. NPA was the first organization to offer a third-party GMP certification program for the manufacturing of dietary supplements and dietary ingredients. NPA also maintains a warning letter database for NPA members to view violations of GMPs.
Most Cited Part 111 Charge as a Function of Year
The chart below includes the top 10 most cited Part 111 citations issued for each year, with the top 5 citations for 2018 color coded for year-over-year comparison. Notable trends based on NPA’s analysis of the data include:
- 21 CFR 111.103 – Beginning in 2015, there was a continual increase in the amount of times this has been cited in FDA-issued warning letters.
- 21 CFR 111.140 – This has shot up to the second most cited subheading of 2018. In the past, it never made it past the top 7 most cited.
- 21 CFR 111.70 – This has consistently been one of the most cited subheadings since 2010. While it is number 3 as of now in 2018, it is important to note that 103 and 140 have both managed to bypass it as a top cited subheading.
- 21 CFR 111.55 – This subheading never even made it to the top 10 until 2017, now it is slowly becoming one of the top cited subheadings.
- 21 CFR 111.105 – This subheading has been slowly gaining traction since 2013, with the exception of it not even making the top 10 chart in 2016.
- 21 CFR 111.503 – The only other time this subheading made it to the top 10 was in 2012.
- 21 CFR 111.75 – Historically, this has been one of the most heavily cited subheadings (if not THE top cited subheading). Interestingly enough, it didn’t even make it to the top 10 this year

Code Definitions:
27 Requirements that apply to equipment and utensils
35 Recordkeeping of equipment and utensils
55 Requirements to implement a production and process control system
65 Requirements for quality control operations when establishing a production and process control system
70 Specifications to establish when establishing a production and process control system
73 Responsibility for determining whether established specifications are met for establishing a production and process control system
75 Determining if specifications are met for establishing a production and process control system
77 Protocol for unmet established specifications for production and process control systems
83 Requirements for reserve samples when establishing a production and process control system
95 Recordkeeping when establishing a production & process control system
103 Written procedures for production and process control systems
105 Quality control personnel responsibilities for production and process control systems
123 Quality control operations that are required for the master manufacturing record, the batch production record, and manufacturing operations
140 Recordkeeping requirements for production and process control systems
205 Requirement to establish a master manufacturing record for each formulation of a dietary supplement
210 Requirements for the Master Manufacturing Record: What to include in master manufacturing record
255 Requirement to establish a batch production record
260 Production and Process Control System: Requirements for the Batch Production Record - Batch record inclusion requirements
455 Requirements that apply to holding components, dietary supplements, packaging, and labels
503 Requirements for written procedures for dietary supplements
535 Recordkeeping for returned dietary supplements
553 Written procedures for product complaints
NPA Educational Webinar
On October 30, 2018 NPA will host FDA officials and industry experts for an educational webinar examining the current trends in GMP compliance and enforcement for dietary supplements. Participants can register online by October 30, 2018. For more information about the webinar, please contact dnissinoff@npanational.org.