08.03.18
A new study, published in the Journal of Gerontology, aimed to evaluate the effect of six months of dietary supplementation with a polyphenol-rich extract from grape and blueberry (PEGB, trademark Memophenol by Activ’Inside) on memory of healthy elderly subjects. The study involved 215 healthy seniors (Mini Mental State Examination score (MMSE) between 26 to 29) aged between 60-70 years old, orally supplemented with 300 mg twice a day of Memophenol.
Learning, short and long-term memories were assessed using validated neuropsychological tests from the Cambridge Neuropsychological Test Automated Battery (CANTAB). Two tests were used before (T0) and after six months (T6) of supplementation:
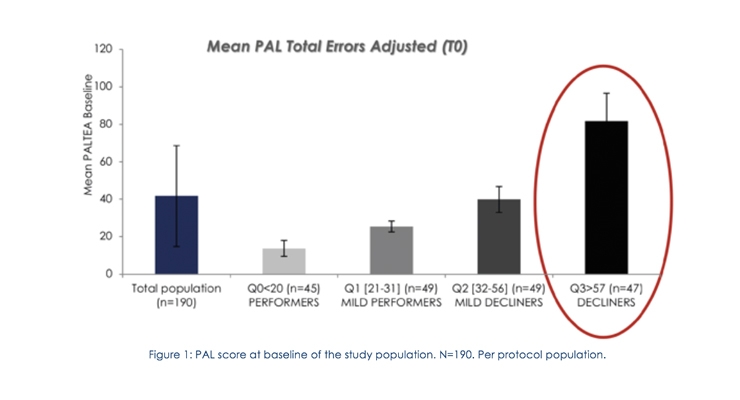
To evaluate how Memophenol could correct a more pronounced memory loss, its effectiveness was evaluated on subjects with the worst PAL score at baseline (decliners) (Figure 1).
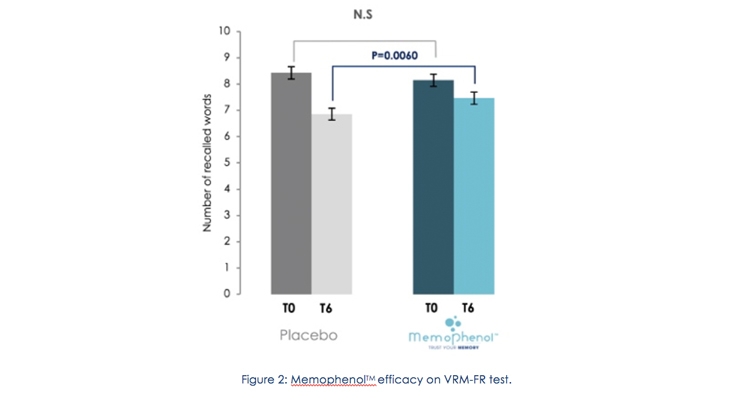
After six months of supplementation, in the total population, the study shows that subjects supplemented with Memophenol significantly improves their Verbal Free Recall Recognition Memory (VRM-FR, p=0.006) scores (Figure 2).
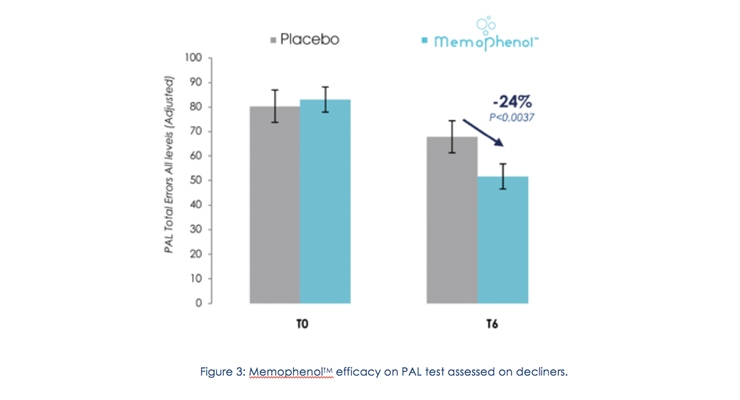
In addition, the subjects having the most prominent cognitive decline at baseline (t=0), also called “decliners,” significantly reduced the number of errors on the PAL test (p=0.037), thus highlighting episodic memory improvements. This decrease is associated to 24% less errors versus placebo (Figure 3).
Finally, the new publication shows that these cognitive improvements are interestingly associated with higher urinary concentration specific flavan-3-ols metabolites in the Memophenol group, confirming that Memophenol supplementation improves memory on seniors with a high bioavailability.
“This publication is a new piece of the Memophenol puzzle. Indeed, the fruit of a four year-international research program, Memophenol is supported by not less than 10 publications and one patent,” said Benoit Lemaire, chief executive officer at Activ’Inside.
Learning, short and long-term memories were assessed using validated neuropsychological tests from the Cambridge Neuropsychological Test Automated Battery (CANTAB). Two tests were used before (T0) and after six months (T6) of supplementation:
- Verbal Recall Memory (VRM) test, assessing short-term memory: 18 words are shown one by one on a screen. People are then asked to recall as many words as possible, immediately (i.e.1 min) following the presentation.
- The Paired Associated Learning (PAL) test, assessing long-term episodic memory. During this test, an increasing number of patterns have to be replaced, depending on the levels.
To evaluate how Memophenol could correct a more pronounced memory loss, its effectiveness was evaluated on subjects with the worst PAL score at baseline (decliners) (Figure 1).
After six months of supplementation, in the total population, the study shows that subjects supplemented with Memophenol significantly improves their Verbal Free Recall Recognition Memory (VRM-FR, p=0.006) scores (Figure 2).
In addition, the subjects having the most prominent cognitive decline at baseline (t=0), also called “decliners,” significantly reduced the number of errors on the PAL test (p=0.037), thus highlighting episodic memory improvements. This decrease is associated to 24% less errors versus placebo (Figure 3).
Finally, the new publication shows that these cognitive improvements are interestingly associated with higher urinary concentration specific flavan-3-ols metabolites in the Memophenol group, confirming that Memophenol supplementation improves memory on seniors with a high bioavailability.
“This publication is a new piece of the Memophenol puzzle. Indeed, the fruit of a four year-international research program, Memophenol is supported by not less than 10 publications and one patent,” said Benoit Lemaire, chief executive officer at Activ’Inside.