By Merle Zimmermann, PhD, American Herbal Products Association (AHPA)01.05.18
Data from Food and Drug Administration (FDA) inspections of dietary supplement facilities conducted in 2016 and 2017 show that FDA continues to inspect an increasing number of facilities annually, and the industry continues to get better at communicating compliance with current good manufacturing practice (cGMP) requirements to FDA inspectors.
The American Herbal Products Association (AHPA) maintains an extensive repository of inspection data that includes FDA 483 inspection reports, inspector observation forms, establishment inspection reports (EIRs), and warning letters.
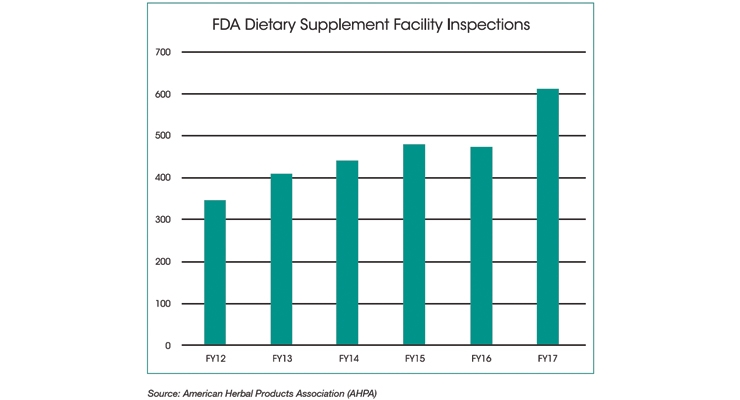
FDA has inspected a total of 2,137 unique dietary supplement facilities (including 184 foreign facilities) between January 2010 and September 2017. The latest data show that FDA inspected 610 dietary supplement facilities in fiscal year (FY) 2017, up from roughly 475 in both FY 2016 and FY 2015. Among the 1,953 domestic inspections, 52% (1,015) of the most recent FDA inspections resulted in no recorded observations or FDA Form 483s.
Since 2011, FDA has increased inspections of all facilities. Implementation of the Food Safety Modernization Act (FSMA) is reflected in an increasing number of dietary supplement facility inspections and re-inspections. Of the 35% of facilities visited more than once, more than half of those (18% of all inspected facilities) were revisited by inspectors within two years of the previous inspection. Under FSMA, FDA’s goal is to inspect “at least” every five years, or every three years for facilities the agency deems are “high risk.”
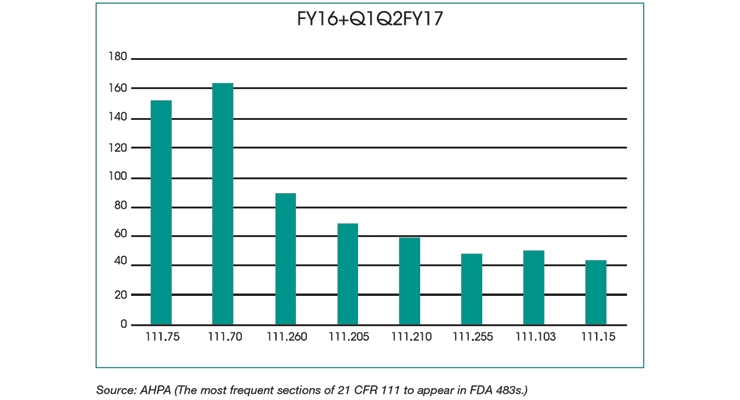
While the data suggest that FDA inspectors are finding more facilities in compliance with cGMP requirements (21 CFR 111), some sections still frequently appear in FDA Form 483s. The most frequent sections of 21 CFR 111 to appear in FDA 483s were:
Specifications
Master Manufacturing & Batch Production Records
Written Procedures for Quality Control
Sanitation Requirements
Immediate investigation and intervention occasionally reduces an observation into a discussion item. In these cases, actions taken during inspection were discussed prior to, or at closeout with inspector. These discussions were recognized and recorded by FDA staff in their establishment inspection reports. Keeping this in mind helps facilities develop a “ladder” of intervention strategies.
1. Making Immediate Changes
Conditions that can be fully addressed during an inspection and shown to the inspector prior to closeout can result in a discussion item on an EIR rather than an observation on an FDA Form 483.
2. Providing a Clear Path to a Solution
If it is impossible to correct a condition observed by an FDA inspector immediately and directly, preparing and scheduling the implementation of new procedures (including appropriate staff training) to remedy the circumstances may effectively address a potential observation, and should help if it does end up appearing as an observation.
3. Making a Commitment During an Inspection
Showing that the solution is coming helps. Providing a schedule and plan for addressing a problem demonstrates an active engagement and a clear road and commitment to improvement. Following through, regardless of whether a potential problem appears as a discussion item on an EIR or an observation on an FDA Form 483, is essential.
Implement commitments prior to the next re-inspection, and follow any timelines as proposed as much as possible. FDA sometimes revisits facilities within a few months to check on progress. Seven percent of facilities are revisited within one year of their previous inspection, and more than half of those inspected more than once are seen by FDA again within two years.
Resource Allocation
The data from recent FDA inspections of dietary supplement facilities can help the industry efficiently allocate compliance resources to effectively communicate how facilities are complying with cGMP requirements when FDA inspectors visit.
If you are interested in more detailed analysis of cGMP inspection data, check out AHPA’s recent webinar: http://bit.ly/2BYP7Gb.
Merle Zimmermann, PhD
American Herbal Products Association (AHPA)
Merle Zimmermann, PhD, is chief information analyst, at the American Herbal Products Association (AHPA). He maintains and updates several online resources that serve the herbal products industry, including an online searchable database of new dietary ingredients submitted to the FDA and the online version of AHPA’s Botanical Safety Handbook. Dr. Zimmermann is currently working to expand the Botanical Identity References Compendium, a centralized source of information containing examples and techniques that have been successfully applied to authenticate and qualify selected botanical materials. Dr. Zimmermann earned his PhD in Chemistry from the University of Maryland.
The American Herbal Products Association (AHPA) maintains an extensive repository of inspection data that includes FDA 483 inspection reports, inspector observation forms, establishment inspection reports (EIRs), and warning letters.
FDA has inspected a total of 2,137 unique dietary supplement facilities (including 184 foreign facilities) between January 2010 and September 2017. The latest data show that FDA inspected 610 dietary supplement facilities in fiscal year (FY) 2017, up from roughly 475 in both FY 2016 and FY 2015. Among the 1,953 domestic inspections, 52% (1,015) of the most recent FDA inspections resulted in no recorded observations or FDA Form 483s.
Since 2011, FDA has increased inspections of all facilities. Implementation of the Food Safety Modernization Act (FSMA) is reflected in an increasing number of dietary supplement facility inspections and re-inspections. Of the 35% of facilities visited more than once, more than half of those (18% of all inspected facilities) were revisited by inspectors within two years of the previous inspection. Under FSMA, FDA’s goal is to inspect “at least” every five years, or every three years for facilities the agency deems are “high risk.”
While the data suggest that FDA inspectors are finding more facilities in compliance with cGMP requirements (21 CFR 111), some sections still frequently appear in FDA Form 483s. The most frequent sections of 21 CFR 111 to appear in FDA 483s were:
Specifications
- 111.75: How a facility determines whether specifications are met
- 111.70: Specifications that must be established
Master Manufacturing & Batch Production Records
- 111.205: The requirement to establish a master manufacturing record
- 111.210: What the master manufacturing record must include
- 111.255: The requirement to establish a batch production record
- 111.260: What the batch record must include
Written Procedures for Quality Control
- 111.103: Requirements for written procedures
Sanitation Requirements
- 111.15: Sanitation requirements applying to the physical plant and grounds
Immediate investigation and intervention occasionally reduces an observation into a discussion item. In these cases, actions taken during inspection were discussed prior to, or at closeout with inspector. These discussions were recognized and recorded by FDA staff in their establishment inspection reports. Keeping this in mind helps facilities develop a “ladder” of intervention strategies.
1. Making Immediate Changes
Conditions that can be fully addressed during an inspection and shown to the inspector prior to closeout can result in a discussion item on an EIR rather than an observation on an FDA Form 483.
2. Providing a Clear Path to a Solution
If it is impossible to correct a condition observed by an FDA inspector immediately and directly, preparing and scheduling the implementation of new procedures (including appropriate staff training) to remedy the circumstances may effectively address a potential observation, and should help if it does end up appearing as an observation.
3. Making a Commitment During an Inspection
Showing that the solution is coming helps. Providing a schedule and plan for addressing a problem demonstrates an active engagement and a clear road and commitment to improvement. Following through, regardless of whether a potential problem appears as a discussion item on an EIR or an observation on an FDA Form 483, is essential.
Implement commitments prior to the next re-inspection, and follow any timelines as proposed as much as possible. FDA sometimes revisits facilities within a few months to check on progress. Seven percent of facilities are revisited within one year of their previous inspection, and more than half of those inspected more than once are seen by FDA again within two years.
Resource Allocation
The data from recent FDA inspections of dietary supplement facilities can help the industry efficiently allocate compliance resources to effectively communicate how facilities are complying with cGMP requirements when FDA inspectors visit.
If you are interested in more detailed analysis of cGMP inspection data, check out AHPA’s recent webinar: http://bit.ly/2BYP7Gb.
Merle Zimmermann, PhD
American Herbal Products Association (AHPA)
Merle Zimmermann, PhD, is chief information analyst, at the American Herbal Products Association (AHPA). He maintains and updates several online resources that serve the herbal products industry, including an online searchable database of new dietary ingredients submitted to the FDA and the online version of AHPA’s Botanical Safety Handbook. Dr. Zimmermann is currently working to expand the Botanical Identity References Compendium, a centralized source of information containing examples and techniques that have been successfully applied to authenticate and qualify selected botanical materials. Dr. Zimmermann earned his PhD in Chemistry from the University of Maryland.