03.13.20
The U.S. Food and Drug Administration (FDA) launched a campaign to help consumers use the new Nutrition Facts Label that appears on packaged foods to make more conscious dietary decisions. The new tagline, “What’s In It For You?,” aims to reach the general public and also focuses on consumers at increased risk of nutrition-related chronic diseases, including obesity.
The campaign includes educational materials of people dressed as food products, modeling their new looks (updated labels) on a fashion runway after receiving a makeover.
“This campaign highlights that the new Nutrition Facts label has been designed to assist consumers in making better informed food choices,” Susan Mayne, PhD, director of the FDA’s Center for Food Safety and Applied Nutrition, said. “If a consumer wants to know how many calories there are in a serving, that information is now highlighted. If a consumer wants to choose a food with more vitamin D or less added sugars, that information is now right there on the label.”
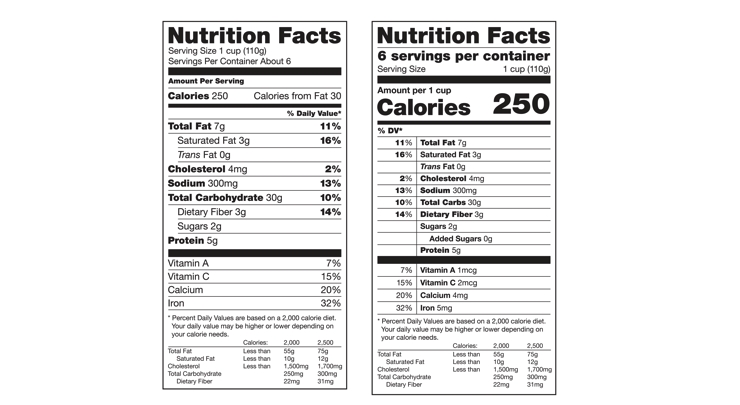
Key changes to the new label include bold listings for serving sizes and calorie counts. Additionally, FDA will require new listings for added sugars, vitamin D, and potassium. A dual column version of the label will be required for food packages that contain 2-3 servings, which FDA determines could be reasonably consumed at one time. On the dual column label, one column lists nutritional facts for a single serving, and the other column lists nutritional facts for the contents of the entire package.
This is the first Nutrition Facts label redesign seen in over 20 years. It is based on scientific information including the link between diet and chronic diseases such as obesity and heart disease. Serving sizes have also been updated to reflect the changes in the amount of food and beverages people tend to consume.
This is one component of FDA’s multi-year Nutrition Innovation Strategy, which is designed to better inform consumers and facilitate innovation toward healthier foods. Aspects of the campaign have also been developed for health care professionals, teachers, dietitians, and community leaders, who can relay information to the public.
Companies with $10 million or more in annual food sales were required to begin using the new label on their products. For manufacturers with less than $10 million in sales, the new label deadline is Jan. 1, 2021.
For some additional insights that can be provided to consumers, the Council for Responsible Nutrition, an ingredient and supplement industry trade group, has also launched an independent public education campaign called Label Wise.
The Label Wise campaign helps keep consumers in the loop on FDA changes made specifically to dietary supplement labels in 2016, which were enacted this year, to help consumers understand changes made to daily recommended values, suggested uses, and other information reflecting the latest developments in nutritional science and FDA standards.
In addition to a two-minute video intended for consumers, the Label Wise website features a consumer fact sheet, an infographic, an interactive “how-to” guide for reading a label, and a stakeholder toolkit.
Ultimately, consumers, FDA, and the food/supplement industry alike stand to benefit from the new label changes and a more knowledgeable consumer base, as they provide the most updated information related to dietary changes, emerging scientific research, and other consumer habits which can lead either to healthy choices or chronic metabolic diseases.
The campaign includes educational materials of people dressed as food products, modeling their new looks (updated labels) on a fashion runway after receiving a makeover.
“This campaign highlights that the new Nutrition Facts label has been designed to assist consumers in making better informed food choices,” Susan Mayne, PhD, director of the FDA’s Center for Food Safety and Applied Nutrition, said. “If a consumer wants to know how many calories there are in a serving, that information is now highlighted. If a consumer wants to choose a food with more vitamin D or less added sugars, that information is now right there on the label.”
Key changes to the new label include bold listings for serving sizes and calorie counts. Additionally, FDA will require new listings for added sugars, vitamin D, and potassium. A dual column version of the label will be required for food packages that contain 2-3 servings, which FDA determines could be reasonably consumed at one time. On the dual column label, one column lists nutritional facts for a single serving, and the other column lists nutritional facts for the contents of the entire package.
This is the first Nutrition Facts label redesign seen in over 20 years. It is based on scientific information including the link between diet and chronic diseases such as obesity and heart disease. Serving sizes have also been updated to reflect the changes in the amount of food and beverages people tend to consume.
This is one component of FDA’s multi-year Nutrition Innovation Strategy, which is designed to better inform consumers and facilitate innovation toward healthier foods. Aspects of the campaign have also been developed for health care professionals, teachers, dietitians, and community leaders, who can relay information to the public.
Companies with $10 million or more in annual food sales were required to begin using the new label on their products. For manufacturers with less than $10 million in sales, the new label deadline is Jan. 1, 2021.
For some additional insights that can be provided to consumers, the Council for Responsible Nutrition, an ingredient and supplement industry trade group, has also launched an independent public education campaign called Label Wise.
The Label Wise campaign helps keep consumers in the loop on FDA changes made specifically to dietary supplement labels in 2016, which were enacted this year, to help consumers understand changes made to daily recommended values, suggested uses, and other information reflecting the latest developments in nutritional science and FDA standards.
In addition to a two-minute video intended for consumers, the Label Wise website features a consumer fact sheet, an infographic, an interactive “how-to” guide for reading a label, and a stakeholder toolkit.
Ultimately, consumers, FDA, and the food/supplement industry alike stand to benefit from the new label changes and a more knowledgeable consumer base, as they provide the most updated information related to dietary changes, emerging scientific research, and other consumer habits which can lead either to healthy choices or chronic metabolic diseases.